Nilotinib Clinical Trials: What They Mean for the Future of Leukemia Treatment
Introduction to Nilotinib and its Impact on Leukemia Treatment
As a passionate advocate for advancements in the medical field, I am excited to discuss the latest developments in leukemia treatment, specifically the Nilotinib clinical trials. Leukemia is a type of cancer that affects the blood and bone marrow, and it currently has no cure. However, recent advancements in targeted therapy, including the use of Nilotinib, have shown promising results in the fight against this disease. In this article, I will delve into the Nilotinib clinical trials and what they mean for the future of leukemia treatment.
Understanding the Mechanism of Nilotinib
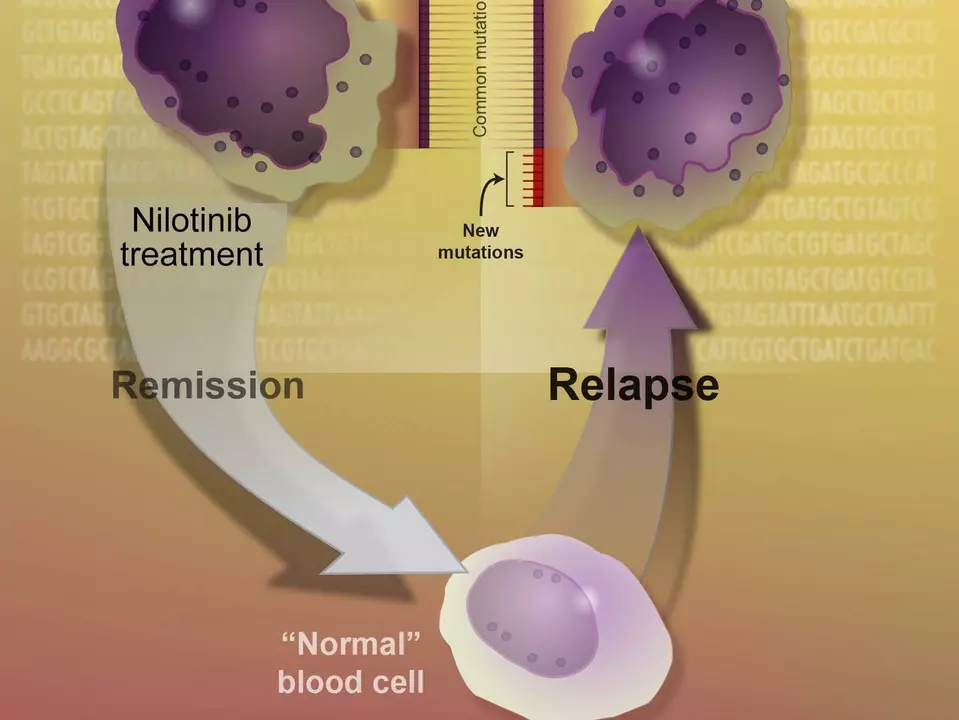
Before discussing the clinical trials, it is essential to understand how Nilotinib works. Nilotinib is a tyrosine kinase inhibitor (TKI) that targets the BCR-ABL protein, which is responsible for the growth and division of leukemia cells. By inhibiting this protein, Nilotinib effectively stops the growth and spread of cancerous cells, allowing for a more targeted approach to treatment. This method differs from traditional chemotherapy, which attacks both cancerous and healthy cells, often leading to severe side effects.
Nilotinib in Comparison to Imatinib
Imatinib, another tyrosine kinase inhibitor, has been the standard treatment for chronic myeloid leukemia (CML) since its approval in 2001. However, Nilotinib has shown to be more potent and selective in targeting the BCR-ABL protein, which has led to its investigation as a potential first-line treatment for CML. The Nilotinib clinical trials aimed to compare the effectiveness and safety of these two drugs, ultimately determining the potential of Nilotinib to replace Imatinib as the standard treatment for newly diagnosed CML patients.
Results from the Phase 2 and Phase 3 Clinical Trials
The results from the Phase 2 and Phase 3 clinical trials have been encouraging. In the Phase 2 trial, Nilotinib demonstrated a higher rate of major molecular response (MMR) compared to Imatinib, with fewer side effects. The Phase 3 trial, known as ENESTnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials - Newly Diagnosed Patients), compared the efficacy of Nilotinib and Imatinib in over 800 newly diagnosed CML patients. The results showed that Nilotinib was more effective than Imatinib in achieving MMR and complete cytogenetic response (CCyR), with fewer instances of disease progression.
Long-term Outcomes and Safety of Nilotinib
The long-term outcomes of Nilotinib treatment have also been promising. In a follow-up study conducted five years after the completion of the ENESTnd trial, Nilotinib continued to show higher rates of MMR and CCyR compared to Imatinib. Additionally, the safety profile of Nilotinib remained favorable, with most side effects being manageable and reversible. This long-term data supports the continued use of Nilotinib as an effective and safe treatment option for newly diagnosed CML patients.
Nilotinib's Potential Impact on Leukemia Treatment
The positive results from the Nilotinib clinical trials have significant implications for the future of leukemia treatment. The trials have demonstrated that Nilotinib is not only more effective than Imatinib in achieving MMR and CCyR, but it also has a more favorable safety profile. This means that Nilotinib has the potential to become the new standard treatment for newly diagnosed CML patients, ultimately improving their chances of achieving long-term remission and increasing their overall quality of life.
Conclusion: The Future of Leukemia Treatment with Nilotinib
In conclusion, the Nilotinib clinical trials have shown that this drug has the potential to revolutionize leukemia treatment, particularly for newly diagnosed CML patients. With higher rates of MMR and CCyR and a better safety profile, Nilotinib offers a promising alternative to the currently used Imatinib. As a passionate advocate for medical advancements, I am excited about the potential impact of Nilotinib on the lives of leukemia patients and look forward to further developments in this field.





Written by Jakob Fitzroy
My name is Jakob Fitzroy, and I am an expert in pharmaceuticals with a passion for writing. I have dedicated my life to studying medication and understanding how it affects various diseases. My goal is to educate people about the importance of proper drug therapy and prevention methods. I have authored numerous articles, providing valuable insights on medication, its development, and its impact on patients. My driving force is to contribute to the ongoing fight against diseases and improve the overall health and well-being of people around the world.
All posts: Jakob Fitzroy